 In Clarksville, Tennessee, an 11-year-old boy was able to grip things for the first time in his life. He was born with missing limbs and body parts. Thanks to 3D printing, his hand was remediated.
In Clarksville, Tennessee, an 11-year-old boy was able to grip things for the first time in his life. He was born with missing limbs and body parts. Thanks to 3D printing, his hand was remediated.
3D printing is responsible for many medical miracles. It has much potential to save lives and provide these kinds of medical materials on the spot. However, the Food and Drug Administration (FDA), always watchful and careful to scrutinize the fabrication of anything used by humans in a medical capacity, could become obtrusive in such oversight when a positive outcome is needed.
The 11-year-old boy’s body parts are critical; anyone would hate to see the cumbersome approval process of the FDA prevail in the fabrication and manufacture of fingers and a hand for him to use.
The FDA’s Guidance on Additive Manufacturing
The FDA is mindful of additive manufacturing, sometimes referred to in their regulatory lexicon as “advanced manufacturing.” It is also working hard to shape existing policy for advanced manufacturing used in the fabrication of medical devices and in a public health emergency. There are particular concerns when 3D printing might be used in an urgent situation, where there’s not time for long approval processes.
When it comes to public health emergency preparedness and response, time and availability of resources are both precious commodities. That’s why the Food and Drug Administration has heightened its focus on advanced manufacturing.
Advanced manufacturing refers to the development of new medical products and their manufacture that ultimately improve things such as:
- Drug quality
- The availability of medicines and treatment
- The time that it takes for equipment and drugs (and other supplies) to reach the needed market
Former FDA Commissioner Scott Gottlieb started the pace in 2017 when he stated:
We’re working to provide a more comprehensive regulatory pathway that keeps pace with those advances, and helps facilitate efficient access to safe and effective innovations that are based on these technologies.
In order to achieve this, the FDA is focused on production methods that may not have yet been explored. They want to develop such methods and bring them to practice so that they can be used to increase emergency preparedness when it comes to public health issues.
3D Printing Medical Devices and Products
3D printing is a significant part of this effort. It enables rapid-scale manufacturing of things that support vaccines and other medical countermeasures to respond to threats and health emergencies such as pandemic influenza or the recent coronavirus. In addition, 3D printing will help shorten the supply chains and increase what the FDA calls “manufacturing resilience.”
This strategy will avoid disruption by public health emergencies and other threats such as natural disasters that are created by unforeseen circumstances. It allows them to create a network of small manufacturing sites that are actually available upon demand when a natural disaster or public health emergency occurs. It also serves as a reserve or overflow capacity for established and centralized manufacturing facilities that might not be able to handle the demands made by a national emergency.
In December 2017, the FDA became the first regulator of 3D printing of medical products. This regulation involved the provision of a comprehensive technical framework for manufacturers to utilize for medical devices.
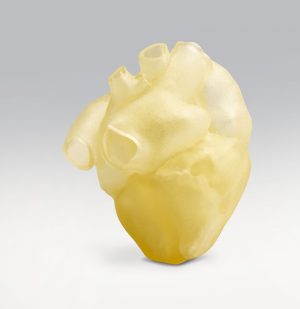
The Stratasys J750 Digital Anatomy 3D Printer has the power to create the look, feel, and function of real organs.
The FDA’s additive manufacturing of medical products core research facility is a collaboration of different stakeholders. It augments and houses complex 3D printing equipment, software, and other technologies used throughout the agency to perform research using this advanced technology.
3D Printing Regulations
The FDA has stepped up its game to make sure that it has very astute regulatory oversight of additive manufacturing — in advance — so that methods may be used in short order when crisis strikes. Because the reality is this: the utility of 3D printing to fabricate medical devices, and the growing application of 3D printing in the manufacturer of medical devices (such as orthotics) is happening.
According to their website, the FDA is “taking many steps to help realize the potential of advanced manufacturing, including issuing guidance on emerging technologies and working with medical product sponsors to clarify regulatory and data requirements necessary to support product applications using advanced manufacturing technologies.”
Consequently, as 3D printing advances, we’ll need to follow the actions of the FDA to regulate and concurrently promote the merits of 3D printing to meet world demand.
Did you know that GrabCAD Print is the medical 3D printing software for the Stratasys Digital Anatomy Printer? Learn how you can start printing realistic human anatomical models today!



Be the first to post a comment.