The Facts
“The shipped product fully corresponds to the product documentation.”
A matter of course?
Or do you have doubts?
The documentation of product-related data in the pharmaceutical industry is generally very extensive. The process gets even more complex if several departments, sites, and potentially external partners are involved. It takes years from the first formulation to the marketing authorization of a drug product. The long time frame makes a consistent and complete documentation even more difficult. Changes are frequent already before the initial submission: new analytical methods, production process adjustments, supplemental data from clinical studies. Once the product is on the market, obviously the changes do not stop. The effects can be disastrous when the numerous and stringent health authority regulations have not been followed accurately. In the worst case, the product cannot be shipped anymore until the problem or inconsistency has been resolved.
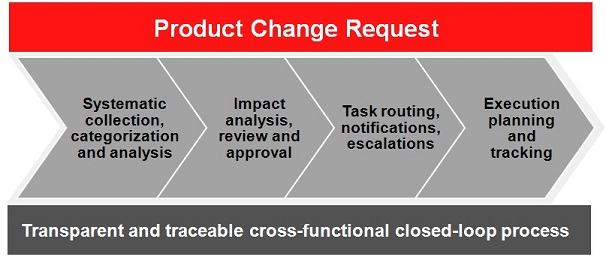
Status Quo
In spite of this situation, cross-functional change control is still handled with Excel sheets and through emails in many companies. Requested, planned, ongoing, and completed changes are not entered and tracked centrally. An excipient is changed in the chemical development, but the technical writer who is currently revising the leaflet text is not aware. Quality managers are frequently forced to approve change requests or documents without having access to all relevant information. The process is not transparent: even if all required persons have provided their signature, the reasons for approving or rejecting are not recorded and cannot be tracked.
Route to Success
There are many software packages that support change management. It gets a bit more difficult when you have to consider the special requirements of the pharmaceutical industry: sophisticated approval workflows; full audit trail, history, and data security that comply with FDA 21 CFR part 11; representation of complex submission or product launch projects, to name just a few. However, the goal is not reached just with suitable software. Change management within the organization is at least as important as a proper tool. Existing walls between departments must be broken down successively both in terms of electronic data exchange and in people’s minds. This cannot be achieved without proactive executive sponsorship. Furthermore, a software implementation for such a purpose is not just an IT project; it should be primarily driven by the business.
Even if the software is flexible enough to represent existing business processes, the introduction of a new central system provides the opportunity to review, simplify and standardize workflows. In many cases, processes were initially designed a long time ago and some workflow steps are done only because of the historic manual and paper-based conditions. Such transformations cannot be achieved over night; a central comprehensive change control platform is the long-term goal which should be approached in manageable phases. Preferably, an area with concrete pains is chosen as a starting point, e.g. because a bottleneck is apparent, regulatory obligations have to be fulfilled, or a legacy system cannot be maintained anymore. A quick success and return on investment is possible and helps to increase the number of supporters within the company. If the introduction is managed properly, significant cost and time savings as well as increased throughput can be realized in a short timeframe. In parallel, a careful analysis is needed which existing systems should be interfaced or replaced.
How Oracle can help Pharmaceutical Companies
Source:: PLM Cafe


Be the first to post a comment.